Experiments with hydrogels
- plant water storage crystals
Class
practical
In this
activity students investigate plant water storage crystals, a product that
contains hydrogels – polymeric smart materials. The practical work
is fun to do, and the results are clear and easy to see.
Lesson
organisation
To
complete all parts of this experiment takes over an hour. If lessons are
shorter than that then part 1 can be done in a prior lesson. The crystals keep
for a few days if they are covered in water.
During the
time that the crystals have to be left, other Experiments with hydrogels, using hair gel and disposable nappies, could
be carried out.
It is a
good idea to ask students to make detailed observations of each part of the
experiment.
The water
crystals can be coloured with a few drops of food colouring (for wonderful,
lurid colours), with strong tea solution (which stains some containers but
provides a useful model of a drug delivery system – see teaching notes) or not
at all (which seems a bit of a shame as they look great when coloured.)
Chemicals
Each working group requires:
Water
crystals, about 100 cm3
Either
strong tea, 500 cm3, or a few drops of food colouring
(optional)
Sodium
chloride (table salt) solution, very concentrated or saturated, 200 cm3
Distilled
water, 400 cm3
Sugar, 1
spoonful
Apparatus
Eye
protection
Each working group requires:
Large ice
cream tub or similar container (at least 1 dm3)
Beakers
(250 cm3), 3
Dessert
spoon or similar measure
White
paper - to place under beakers to see what is happening more easily
Stirring
rods, 3
Petri
dishes – lids not required, 2
Access to:
Sieve
(the plastic ones used for sifting flour are fine) or large funnel and either
paper towels or filter paper
Tea
strainer (only required if a funnel is used earlier, otherwise the sieve can be
used again)
Health
and Safety and Technical
Water
crystals - Water crystals are available from garden centres and are sold under
various names including Phostrogen Swellgel. Each group needs about a
teaspoonful.
Strong
tea - For the strong tea use two tea bags per litre, pour on boiling water and
leave to brew overnight. This tea stains some containers.
Distilled
water - If distilled water is not available, tap water can be used but the
results are not as spectacular.
Procedure
Part 1
a Estimate the volume of one teaspoonful of the water crystals.
a Estimate the volume of one teaspoonful of the water crystals.
b Put
about 500 cm3 of tea, tap water or water coloured with a few
drops of food colouring into the beaker or tub. Add one teaspoonful of water
crystals, stir gently and leave on one side for at least half an hour, or
overnight.
Part 2
a Sieve the water crystal mixture. It is best to do this over a large tub rather than the sink in case you drop it. Wash the gel crystals carefully once or twice in water to remove any excess tea or food colouring if you used it. Estimate the new volume of your crystals.
a Sieve the water crystal mixture. It is best to do this over a large tub rather than the sink in case you drop it. Wash the gel crystals carefully once or twice in water to remove any excess tea or food colouring if you used it. Estimate the new volume of your crystals.
b Stand
the three 250 cm3 beakers on a piece of
white paper.
c Put
two dessert spoons of the gel crystals into each beaker, estimate their volume
and then add about 200 cm3 of salt
solution to one and 200 cm3 of
distilled water to each of the others. Add a spoonful of sugar to one of the
beakers with water in it. Label the beakers.
d Stir
the mixtures gently – using a separate stirring rod for each one so that the
solutions do not become cross-contaminated. Leave for 10–15 mins, stirring
occasionally.
e If
you used tea, pour some of the solution from each beaker into a petri dish
placed on the white paper. Use a tea strainer or sieve to prevent any crystals
getting onto the petri dish. Note carefully the colour of each liquid.
f Sieve
the remaining mixtures, discarding the excess liquid and returning the crystals
to the beakers. Estimate their new volumes.
Teaching
notes
This
activity can be used to enhance the teaching of ionic and covalent bonding, or
hydrogels can be considered as an interesting polymer as well as an example of
a smart material. Hydrogels are smart materials because they change shape when
there is a change in their environment – in this case it is the change in the
concentration of ions.
Students
need to have some knowledge and understanding of ionic and covalent bonding,
reversible reactions, and acids and bases to understand what is happening.
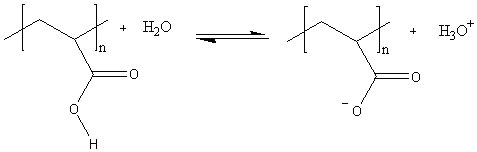
Hydrogels
are polymers that can retain many times their own weight in water. They are
often polymers of carboxylic acids that ionise in water, leaving the polymer
with several negative charges down its length. This has two effects. First, the
negative charges repel each other and the polymer is forced to expand.
Secondly, polar water molecules are attracted to the negative charges. This
increases the viscosity of the resulting mixture still further as the polymer
chain now takes up more space and resists the flow of the solvent molecules
around it.
The
polymer is in equilibrium with the water around it, but that equilibrium can be
disturbed in a number of ways. If the the ionic concentration of the solution
is increased – eg by adding salt – the positive ions attach themselves to the
negative sites on the polymer, effectively neutralising the charges. This
causes the polymer to collapse in on itself again. Adding alkali removes the
acid ions and moves the equilibrium to the right; adding acid has the opposite
effect.
There are
a large number of hydrogels and they are sensitive to different pHs,
temperatures and ionic concentrations. By using a mix of monomers to create the
polymer these characteristics can be fine-tuned.
The
hydrogels that are commonly available and are used in this practical activity
are sensitive to salt concentration, but do not show much change across the pH
range that can be readily investigated in the classroom. However, they do lend
themselves very well to a range of investigative practical work. For example,
their volume in different amounts of water or in different salt concentrations
can be measured. For this type of investigation it is best to use either plant
water crystals or to order sodium polyacrylate from Sigma Aldrich – this has
a smaller crystal size and gives faster results.
Students
should make detailed notes on their experiments, noting changes in volume,
colour and any other observations. Some expected observations could include:
The
crystals swell up from about 5 cm3 to about
500–600 cm3. They take on the colour of the tea (or food
colouring), showing that the tea has also been absorbed.
When
distilled water is added to the hydrated crystals, they swell up further. The
tea remains absorbed in the crystals and the water does not change colour. When
salt water is added to the hydrated crystals, they begin to shrink and the
water changes colour as the tea is released. It is possible to measure the
aproximate size of individual pieces of the hydrogel too, and to show that the pieces
have swollen or shrunk. The hydrated crystals in the sugar solution have the
same volume as the ones in the distilled water. If they are left for up to 15
mins the tea is not released. (After this time, the water in the hydrated
crystals is in equilibrium with the water in the beaker and some tea may begin
to be observed.)
These
observations show that the hydrogel responds to changes in the ionic
concentration of the solution – the salt, which is ionic, causes the hydrogel
to collapse but the covalent sugar does not.
Research
is currently being done to see if it is possible to use hydrogels and similar
materials as a drug delivery system – a way to get drugs and medicines to where
they are required in the body. The experiment with tea and the hydrogel is a
model of this type of drug delivery system. The drug is first loaded onto the
carrier and then it is released at the right location. The tea represents the
drug and the hydrogel is the carrier.
No comments:
Post a Comment